Case studies within animal research
Refinement of a mouse model to study diastolic heart failure

Dr Sarah Walsh, a Research Fellow at Robert Gordon University’s School of Pharmacy, Applied Sciences and Public Health, is leading a study on whether the role of Wnt signalling in diastolic heart failure can be better understood by using a refined model of the disease with the aim of discovering new therapeutic targets.
Heart failure is a complex clinical syndrome with various causes, including previous heart attacks, cardiomyopathies, and hypertension, all of which cause a large burden on the NHS and account for one million bed days per year and 5% of all emergency admissions to hospital.
Diastolic heart failure (DHF) develops in response to conditions such as persistent high blood pressure, (hypertension), which causes the structure of the ventricles of the heart to change due to it having to work harder against the increased pressure in the arteries.
This causes the heart muscle to become thicker (hypertrophy) and stiffer (fibrosis), which reduces the ability of the heart to circulate blood efficiently around the body.
DHF mainly affects, although is not limited to, older women with co-morbidities such as hypertension, obesity and/or Type 2 diabetes mellitus, and accounts for approximately 16% of heart failure cases.
According to the European Society of Cardiology, over the last twenty years the survival rate for patients with DHF has remained unchanged due to the lack of effective treatments, emphasising the importance for the development of new therapies.
Using a refined model of diastolic heart failure
The established mouse model of DHF, that has been used in over 2,000 published studies to date, involves the surgical implantation of an osmotic mini-pump under the skin of the flank of the animal that slowly releases the powerful vasoconstrictor angiotensin II (Ang II).
While this model does recapitulate several aspects of DHF, such as cardiac hypertrophy, fibrosis and impaired relaxation of the heart, these mice can also exhibit depressed systolic (i.e., contractile) function that is not a feature of the disease.
To avoid any invasive surgery, Dr Walsh’s team therefore adopted a refined mouse model of DHF that has been described by a group from the University of Texas Southwestern Medical Center.
This approach involves the administration of both a drug in drinking water, to cause hypertension, and a high fat diet, to cause obesity, which, when combined, results in the development of DHF in these animals.
Funded by an MRC CASE Award in collaboration with Astra Zeneca in Sweden, data from the RGU experiments (depicted in Figure 1) also found that this intervention resulted in the characteristic changes seen in DHF with mice developing cardiac hypertrophy (A), fibrosis (B), impaired diastolic function (C), and maintained systolic function (D).
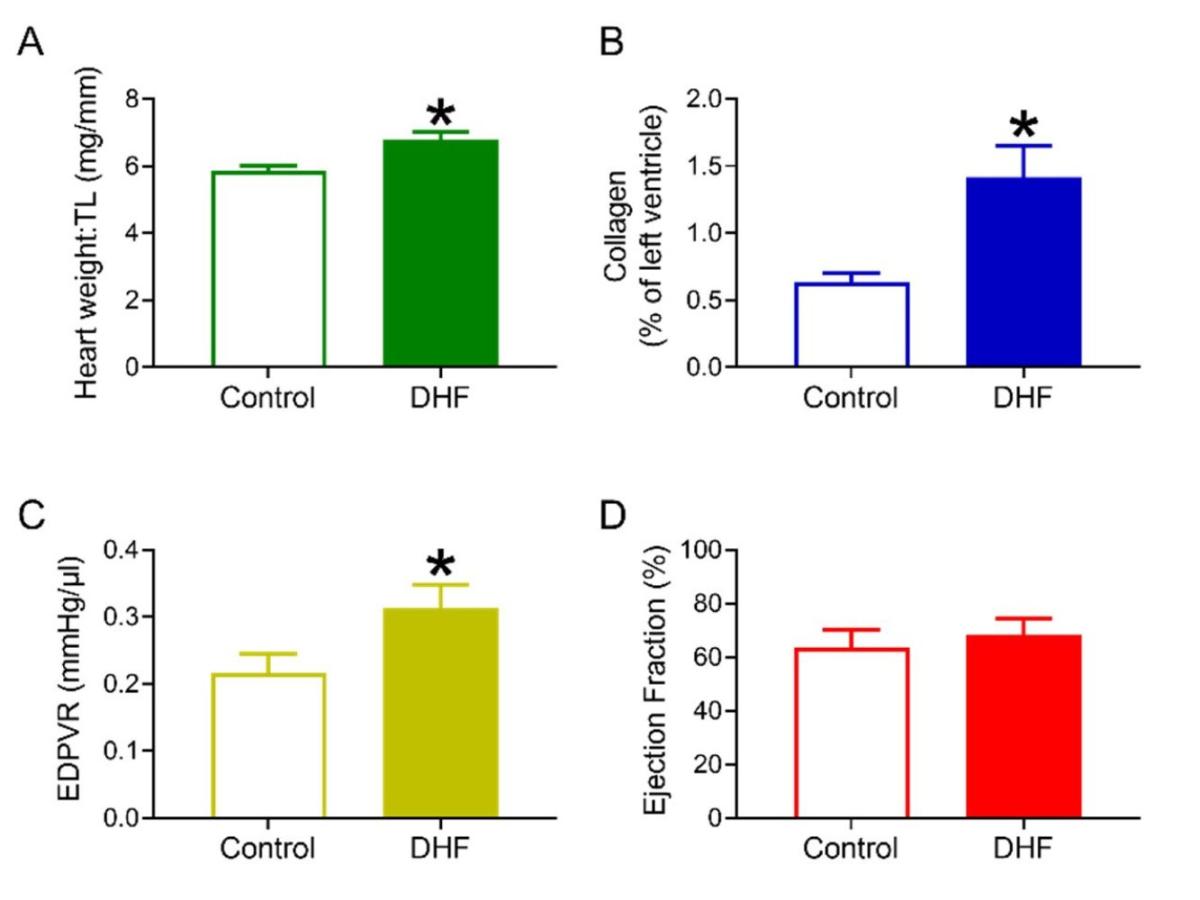 Figure 1. Cardiac structural and functional changes associated with DHF.
Figure 1. Cardiac structural and functional changes associated with DHF.
Targeting Wnt Signalling for the Prevention of DHF
Wnts form a particular group of proteins that have been shown to promote the development of both cancer and cardiovascular disease.
The project at RGU has been exploring whether disruption of the messages that Wnts signal to the cells of the heart has the potential to improve heart function in an experimental model. By using a drug that prevents the release of Wnts, the research has shown that this improves the ability of the heart to relax and fill with blood prior to contraction.
The exact way (or ways) in which this drug intervention improves heart function is unknown, and currently an area being explored. However, the findings suggest it may involve changes in the structure of the heart to increase its compliancy.
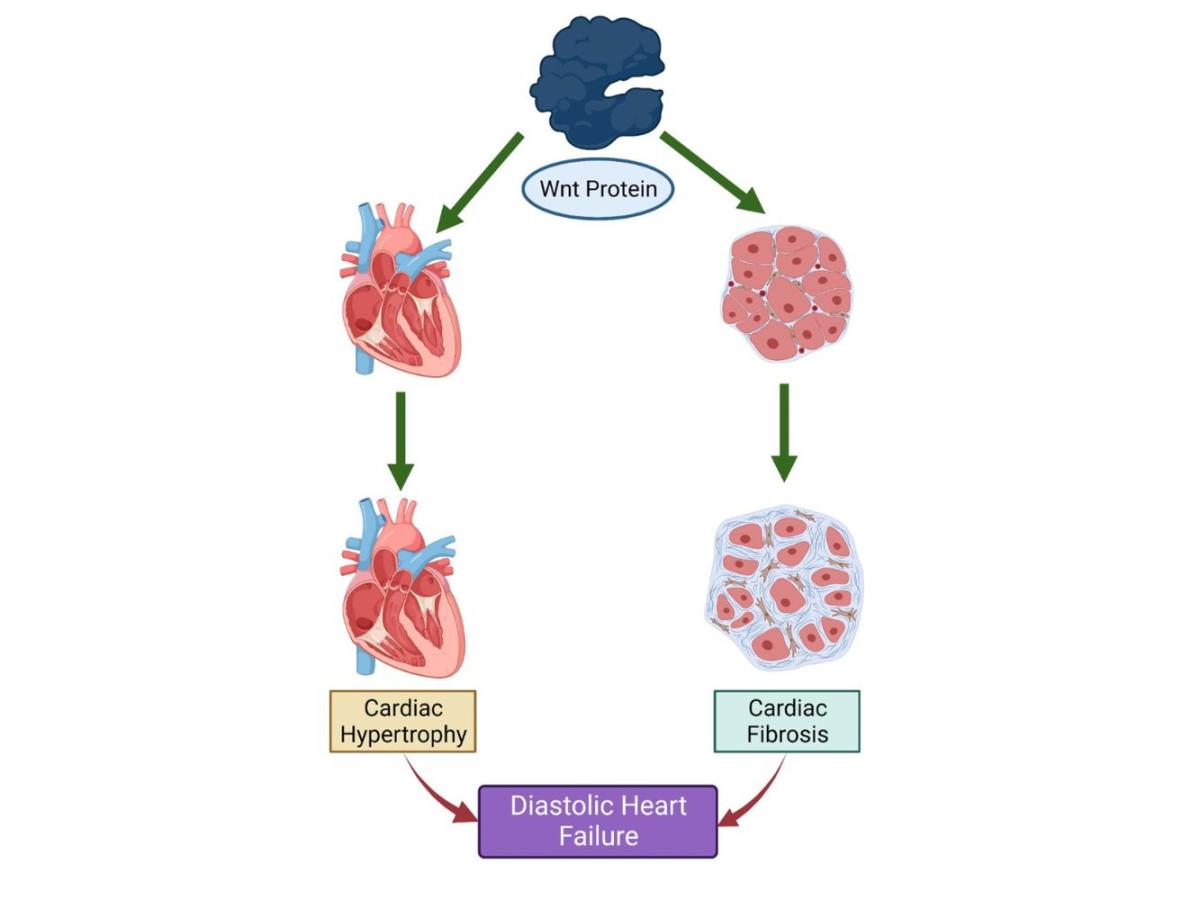 Figure 2. Wnt proteins promote the development of cardiac hypertrophy and fibrosis and consequently DHF
Figure 2. Wnt proteins promote the development of cardiac hypertrophy and fibrosis and consequently DHF
